[ad_1]
Neuralink, a company founded by Elon Musk, has reportedly known for years that an issue could arise with an implant it’s been testing for use in patients with paralysis, according to five sources who did not want to be named due to confidentiality agreements. The issue involves tiny wires dislodging in the patient’s brain. These thin wires were intended to decode brain signals allowing paralyzed patients to operate digital devices with their thoughts.
The startup acknowledged the problem openly last week, however, it had been aware of the potential risk since animal testing took place prior to its US approval last year. Despite this, Neuralink did not consider the risk significant enough to undertake a redesign.
As a result of this electrode issue, the number of working signal-measuring electrodes dropped in Neuralink’s first human trial. Despite this, the implanted device was still able to monitor the patient’s brain signals after the algorithm was altered to increase sensitivity.
Interestingly, the U.S. Food and Drug Administration (FDA) had been informed about the potential issue with the wires, as the company included the animal testing results in its application to start human trials. Despite this, it has yet to comment on its potential significance, instead stating it would continue to monitor patient safety during the study.
Any redesign may prove challenging itself due to risks associated with anchoring the threads in the brain. The implant’s design currently aims to safely remove or dislodge threads, allowing for technology upgrades over time.
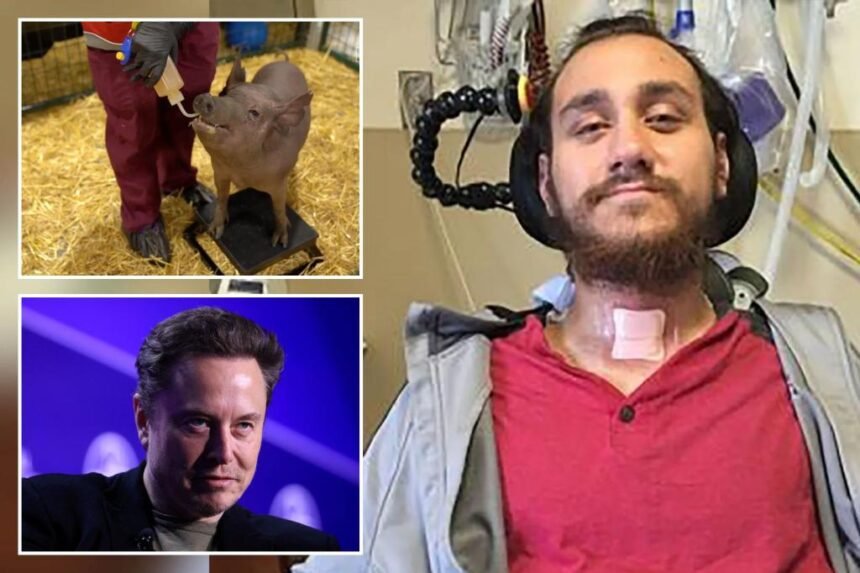
Neuralink’s first implant, which was received by a patient named Noland Arbaugh in January, has nonetheless enabled him to control a computer cursor, browse the internet, and play video games using only his thoughts. Noland, paralyzed from the shoulders down due to a 2016 diving accident, has even surpassed the world record for cursor control speed using his thoughts after the surgery.
Still, questions remain about whether the company should persist with the trials without redesigning the device, especially if more wires detract and the algorithm tweak proves insufficient. Additionally, complications like brain tissue damage could arise if the threads dislodge or if the implant is removed.
[ad_2]